Visit our community sponsor

Thanks:
0

Likes:
0
-
DIY Zinc Plating for Donor Nuts and Bolts
If you have rusty donor parts and have not read the thread on old school electrolytic de-rusting, then do yourself a favor and take a quick look... several forum members have posted pictures that are almost too good to believe... but the process works as advertised.
One problem with electrolytic de-rusting is that the part comes out of the de-rusting bucket with no protective oxide layer, so you must immediately do something to passivate or seal the exposed metal or it will start rusting before your eyes. Painting works great on larger parts, but not so well on nuts and bolts, and certainly not very well on threads. After considerable study, I decided to try home-brew zinc plating.
As it turns out, zinc plating is pretty easy to do at home, with easy to obtain materials that are not particularly dangerous. The washer shown below was my very first try, and developed a completely usable protective coating in about twenty minutes.
DSC_1040.jpg
If you want to give it a try, here's what you will need...
1) A supply of nearly pure zinc metal. I bought a 30 ft long by 2.5 inch wide roll of zinc at my neighborhood home improvement store (about $30). It is sold in the roofing supply isle to keep moss from growing on your roof. But check around first because not all stores have it, and most employees have never heard of it. I verified that a nearby store had it by checking inventory on their web site. They had a little trouble finding it, but since the computer said they had it they kept looking until it was located.
2) A low voltage power supply that can be adjusted from about 0.5 Volts to about 3 Volts. A rheostat can be used to cut the voltage if all you have is a 12 Volt supply.
3) Vinegar from the grocery store. Find a bottle that says "Dilluted to 5% Acidity". Vinegar is actually a mild solution of acetic acid and is what slowly dissolves the zinc metal into solution.
4) Epsom Salt from the grocery store or pharmacy. Epsom salt is Magnesium Sulfate and is the conductive "electrolyte" of the plating solution.
5) Sugar from the grocery store (plain old table sugar). Sugar is the "Brightener" of the plating solution. It actually interferes with the formation of zinc crystals, causing many smaller crystals to form on the surface instead of fewer larger crystals, thereby improving a frosty looking surface to a smoother more reflective one.
6) You will also need several clean plastic containers to mix and store the plating solution and do the actual plating.
The plating solution recipe (which you can scale up or down as you like):
1 liter of vinegar (5%)
100 grams Epsom Salt
120 grams sugar
8 - 10 square inches of zinc pieces
Add the Epsom Salt and sugar to room temperature vinegar and stir until dissolved. Add the zinc pieces and leave lightly covered for 24 hours.
You may not see any bubbling for the first few hours, but by the end of the first day you should see small bubbles coming off the zinc pieces. This is the zinc metal being converted to soluble zinc acetate while liberating hydrogen gas. Don't tightly cap the solution or the pressure from the hydrogen gas will build up and the container may rupture. The longer you wait for the zinc to dissolve, the faster (and some say better) the plating will build up when you start your first run. Once the run starts, you are actually dissolving zinc off the anode at the same rate you are plating zinc onto the cathode, so the zinc in solution should not get depleted.
To actually plate something, make an anode (+ terminal) of zinc metal, and attach the cathode (- terminal) to the part you want zinc plated. The submerged area of the anode should be a little larger than the area of the part being plated.
DSC_1030.jpg
If your solution has a sufficient amount of dissolved zinc acetate then you will see the part start to turn zinc colored almost immediately.
DSC_1032.jpg
The voltage should be adjusted for about 65 mA (milli-Amps) per square inch of part being plated. Of course most of us do not have a milli-ammeter so some experimentation should be expected. The goal is to try different amounts of current and select the setting that gives the nicest looking coating. If the voltage is too high the edges of the part will look uneven, or have a burned look. If the voltage is too low the plating run will take too long and the finish may be more frosted or dull looking. If the voltage is set correctly, only a very slight amount of bubbling will occur. Violent bubbling means the voltage is too high.
After 10 minutes or so remove the part and give it a light rub with a ScotchBright pad to shine it up. If the plating looks too thin you can stick it back into the solution for another run.
It is preferable to do a sequence of several short (10 - 15 minute) runs with a rub between each, than to do one long continuous run in the plating solution. This intermediate polishing step is called "carding" in the plating literature. I don't know why.
The most important preparation step is cleaning the part. The plating will not stick to fingerprint oil, dirt, or any contamination. Most commercial plating shops have very thorough cleaning steps with a final acid etch bath just before the plating run. Scrub the part with detergent, rinse well, and wear gloves to keep the part clean. This is apparently the most common cause of peeling in DIY endeavors.
If you try it, please let us all know how it goes.
Jeff
 Posting Permissions
Posting Permissions
- You may not post new threads
- You may not post replies
- You may not post attachments
- You may not edit your posts
-
Forum Rules

Visit our community sponsor




 Thanks:
Thanks:  Likes:
Likes: 


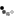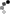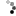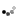
 Reply With Quote
Reply With Quote